Microbiology And Sterility Assurance In Pharmaceuticals And Medical Devices
Get Microbiology and Sterility Assurance in Pharmaceuticals and Medical Devices Books now. An effective sterilization process has a very low Sterility Assurance Level SAL meaning that there is an.
Knowledge regarding the devices bioburden Validation of the sterilization process.
Microbiology and sterility assurance in pharmaceuticals and medical devices. Consultant in the Pharmaceutical Biotech Medical Device manufacturing and testing industries. Download and Read online Microbiology And Sterility Assurance In Pharmaceuticals And Medical Devices ebooks in PDF epub Tuebl Mobi Kindle Book. Eds Microbiology and Sterility Assurance i n Pharmaceuticals and Medical Devices New Delhi.
Read reviews from worlds largest community for readers. WELCOME TO THE LIBRARY. Microbiology and sterility assurance in pharmaceuticals and medical devices Download Microbiology And Sterility Assurance In Pharmaceuticals And Medical Devices ebooks in PDF epub tuebl textbook from SkinvadersCom.
Microbiology And Sterility Assurance In Pharmaceuticals And Medical Devices. Microbiology and Sterility Assurance in Pharmaceuticals and Medical Devices book. Sterile manufacturing facilities especially require state-of-the-art skills to ensure sterility and compliance at all times.
Handbook of Microbiological Quality Control in Pharmaceuticals and Medical Devices. Microbiology and Sterility Assurance in Pharmaceuticals and Medical Devices 9788190646741 by Madhu Raju Saghee. Sterile medical device requirements are defined by national or regional standards and regulations which detail the sterility requirements.
Click Get Books and find your favorite books in. 9788190646741 Microbiology and Sterility Assurance in Pharmaceuticals and Medical Devices Business Horizons Injectable product manufacturing is booming because of the growth of new biopharmaceuticals and small molecule anticancer drugs. Manufacturers of sterile medical devices must demonstrate the microbiological safety of their products by means of.
We cannot guarantee that Microbiology And Sterility. You can read all your books for as long as a. The results of 45 years of scientific and technological development are laid down in these 33 chapters.
Sterilisation is therefore of great importance to healthcare and the manufacturers of medical devices and pharmaceuticals. Amazonin - Buy Microbiology and Sterility Assurance in Pharmaceuticals and Medical Devices book online at best prices in India on Amazonin. Years Employer Title Department.
Download or read online Microbiology and Sterility Assurance in Pharmaceuticals and Medical Devices written by Anonim published by Unknown which was released on 2011. New book issued for microbiology and sterility assurance. These chapters all written by international experts give a vivid picture of todays pharmaceutical microbiology.
Microbiology and Sterility Assurance in Pharmaceuticals and Medical Devices Business Horizons 2011 - Medical instruments and apparatus - 964 pages 2 Reviews. Sterility sterilisation and sterility assurance for pharmaceuticals examines different means of rendering a product sterile by providing an overview of sterilisation methods including heat radiation and filtration. Download full Microbiology And Sterility Assurance In Pharmaceuticals And Medical Devices books PDF EPUB Tuebl Textbook Mobi or read online Microbiology And Sterility Assurance In Pharmaceuticals And Medical Devices anytime and anywhere on any device.
Download full Microbiology And Sterility Assurance In Pharmaceuticals And Medical Devices Book or read online anytime anywhere Available in PDF ePub and Kindle. Tidswell and a great selection of similar New Used and Collectible Books available now at great prices. 173-192 United States Pharmacopoeia 2012.
Get Free Microbiology And Sterility Assurance In Pharmaceuticals And Medical Devices Textbook and unlimited access to our library by created an account. Experts from top pharmaceutical companies like Baxter Johnson and Johnson Amgen Pfizer Patheon Sartorious Gador Catalent British NHS GE Healthcare and many more top experts from industry and academics have come together to create this collection of knowledge of Microbiology as related to Pharmaceuticals Medical Devices and Biotechnology. Sterilization of a medical device may include exposure to ethylene oxide gamma irradiation steam dry heat or chemical sterilization under defined conditions and any necessary post-treatment required for the removal of by-products.
Performs consultation in all areas of Quality Control Assurance Sterility Assurance Aseptic manufacturing and Testing etc. 9788190646741 Microbiology and Sterility Assurance in Pharmaceuticals and Medical Devices Business Horizons Injectable product manufacturing is booming because of the growth of new biopharmaceuticals and small molecule anticancer drugs. Request PDF Microbiology and Sterility Assurance in Pharmaceuticals and Medical Devices This book therefore represents an unparalleled and unprecedented text in the field of pharmaceutical and.
With a view to provide such cutting-edge information within reach of even the SMB pharma sector this book Microbiology and Sterility Assurance in Pharmaceuticals and Medical Devices has been launched. Microbiology and Sterility Assurance Book 2011 - Free download as Word Doc doc docx PDF File pdf Text File txt or read online for free. Microbiology and Sterility Assurance in Pharmaceuticals and Medical Devices by Tim Sandle February 2011 Business Horizons edition Hardcover.
Free delivery on qualified orders. Microbiology and Sterility Assurance in Pharmaceuticals and Medical Devices. Read online Microbiology And Sterility Assurance In Pharmaceuticals And Medical Devices books on any device easily.
Microbiologists working in both the pharmaceutical and medical device industries face considerable challenges in keeping abreast of the myriad. What are you looking for Book Microbiology And Sterility Assurance In Pharmaceuticals And Medical Devices Click Read Now PDF Download Get it for FREE Register 100 Easily. CRC Press Aug 17 2000 - Medical - 280 pages.
Read Microbiology and Sterility Assurance in Pharmaceuticals and Medical Devices book reviews author details and more at Amazonin. Download Microbiology And Sterility Assurance In Pharmaceuticals And Medical Devices Book PDF. Microbiology And Sterility Assurance In Pharmaceuticals And Medical Devices.

Pdf Quality Assurance For Sterile Products

Medical Devices Hygiene Monitoring Through Protein Detection
Sterilisation Methods For Medical Devices And Pharmaceutical Products

Evaluation Of Immunogenicity And Allergic Reactions To Medical Device Materials And Combination Products Pacific Biolabs

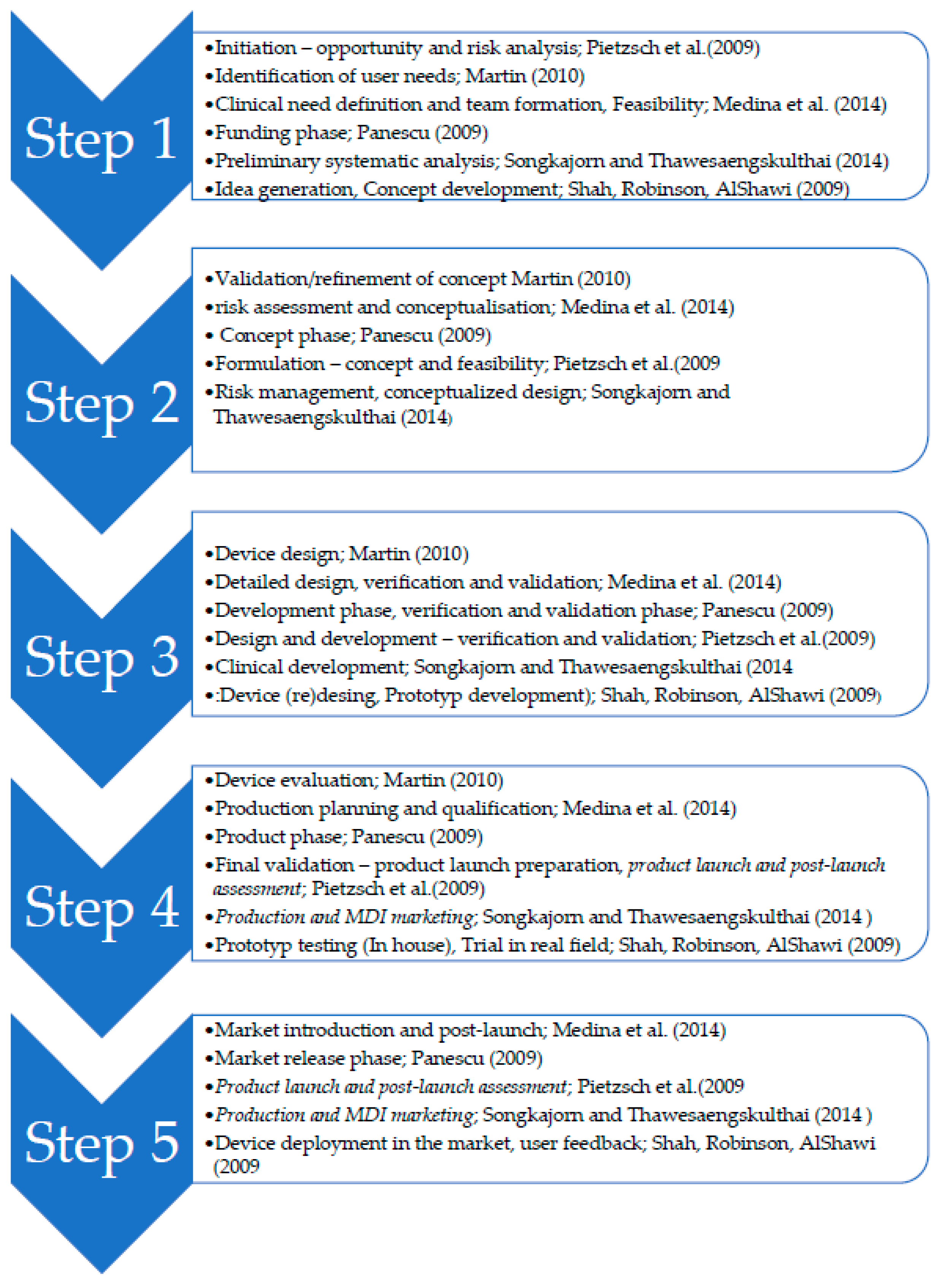
Sustainability Free Full Text Complexity Stage Model Of The Medical Device Development Based On Economic Evaluation Meddee Html

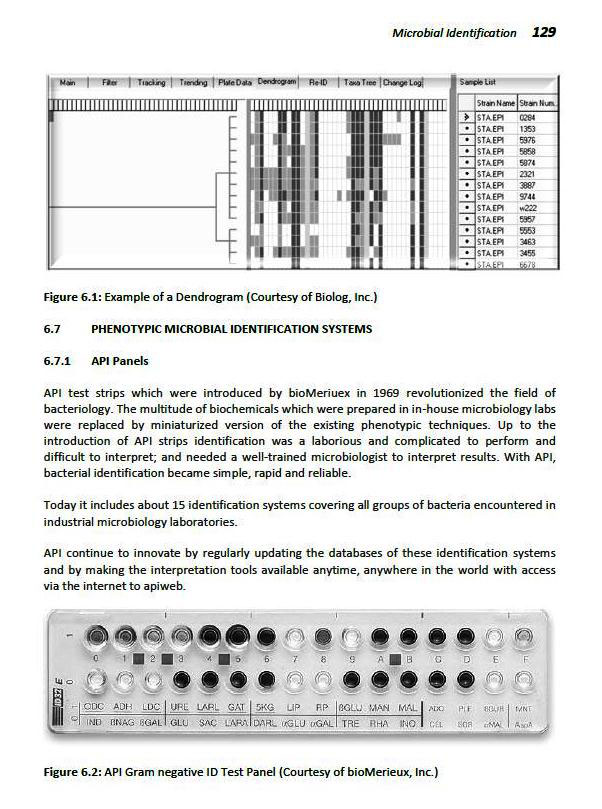
Guide To Microbiological Control In Pharmaceuticals And Medical Devices Second Edition Semantic Scholar

Frontiers Sterility Assurance Across Sectors New Paradigms And Tools Medical Technology

Amazon Com Assurance Of Sterility For Sensitive Combination Products And Materials New Paradigms To Bring Innovative Healthcare Products To Patients 9780128050828 Lambert Byron Lam Stan Hansen Joyce Bryans Trabue D Books

Good Design Practices For Gmp Pharmaceutical Facilities Ebook Rental Pharmaceutical Manufacturing Pharmaceutical Science Books

Cleanrooms Facilities And Practices By Michael Kozicki In Writing Practice Books

Handbook Of Microbiological Quality Control Pharmaceuticals And Medical Devices Book 2000 Worldcat Org
Building A Smart Business Pharmaceutical Medical Device Biotech In The Age Of Iot Ivt

Validation Standard Operating Procedures A Step By Step Guide For

Microbiology And Sterility Assurance In Pharmaceuticals And Medical Devices Madhu Raju Saghee Tim Sandle Edward C Tidswell Madhu Raju Saghee Tim Sandle Edward C Tidswell 9788190646741 Amazon Com Books
Business Horizons Pharmaceutical Publishers Home Facebook

Pharmaceutical Microbiology Essentials For Quality Assurance And Quality Control 1 Sandle Tim Amazon Com

Microbiology And Sterility Assurance In Pharmaceuticals And Medical Devices Madhu Raju Saghee Tim Sandle Edward C Tidswell Madhu Raju Saghee Tim Sandle Edward C Tidswell 9788190646741 Amazon Com Books


Posting Komentar untuk "Microbiology And Sterility Assurance In Pharmaceuticals And Medical Devices"